J. Geo. Chem. Soc., 2021, Vol. 1, Issue: 1, pp. 23 - 26
Syntheses and Researches of 1N,3N-bis(4-(1H-benzo[d]imidazol-1-yl)thiazol-2-yl)naphthalene-1,3-diamine
Ivane Javakhishvili Tbilisi State University
Abstract.
New hetero cyclic system 1N,3N-bis(4-(1H-benzo[d]imidazol-1-yl)thiazol-2-yl)naphthalene-1,3-diamine has been synthesized in stagedprocess transformation of 1,3-di-Aminophenylnaphthalene. Oxo- and Halogen-containing Thiazo-Naphthyl-di-amine systems were synthesized and characterized as Intermediary products. Preparative Method of delivering 1,1'-(naphthalene-1,3-diyl)bis(thiourea) has been processed and optimal conditions for these reactions are determined.
There has also been conducted the condensation of 1,1'-(naphthalene-1,3-diyl)bis(thiourea)with Ethyl Bromine of Acetate with formation of relevant 2,2'-(naphthalene-1,3-diylbis(azanediyl))bis(thiazol-4(5H)-one). Here has also been determined the optimal conditions of the Cyclization reactions. Reactions of Chlorinating 2,2'-(naphthalene-1,3-diylbis(azanediyl))bis(thiazol-4(5H)-one) with Phosphorus Oxychloride were carried out. It has been established that productivity of chlorinating reaction is the highest for initial Oxy compounds Phosphorus Oxychloride and Pyridine in case when taken in equimolar correlation.Condensation of 1N,3N-bis-(4-chlortiazol-2-yl)naphthalene-1,3-diamine with benzimidazole was performed the final compound.
Keywords: Naphthalenediamine, Naphthalene-bis-thiourea, Thiazole, cyclization reactions, chlorination
We carried out the implementation of syntheses of new Naphthalene ring containing biologically active compound which consists of different Hetero cycles. In particular, via staged process, transformation of naphthalenediaminewe have synthesized new di-Thiazonaphthylamine,containing ring of Imidazole. As Intermediary products are characterized naphthalene-bis-thiourea, Oxo- and Halogen-containing systems which in turn are interesting because of their structural likeness to other, well known biologically active compounds.
Studies of naphthalene-bis-thiourea have attracted increasing attention due to their potential use in agriculture insecticidal, herbicidal, and pesticidal activities; Some thiourea derivatives arewidely used in many fields including pharmaceutical industry due to the biological properties such as antimicrobial, antibacterial, antifungal, anticancer, etc.[1-8]. Furthermore, aryl and acyl thiourea derivatives are attractive model compounds for studies in solid-state chemistry due to their tendency to form intra- and intermolecular hydrogen bonds of the N-H proton-donor groups to sulfur and carbonyl oxygen atoms. Recently, compounds of thiourea in coordination with metal complexeshave been reported by many researchers such as NLO materials [9].
Our aim on first stage was to produce Naphthalenediamine di-Thiocarbamide. From publications we are aware of two exit ways of obtaining Thiocarbamide from Aromatic amines: one staged method of syntheses as well as multi staged-we used both these ways. It turned out that in case of 1,3-Naphthalenediamineone stage reaction in progress. Is characterized with low productivity in other words it has low outcome compare to replacing Anilines, mentioned in publications. This can be explained on the huge size of Naphthalene’s nucleus creates steric obstacles.
In order to increase the productivity, outcome of 1,1'-(naphthalene-1,3-diyl)bis(thiourea) (5) we conducted the stageby stage reaction (scheme 1), via interaction of Benzoyl Chloride (1) and Ammonium Thiocyanide in Acetone environment. We at first got intermediary product Benzyl Thiocyanate (2) by interaction of which with1,3-Naphthalenediamine (3) (in molar correlation 2:1) obtained product N,N'-((naphthalene-1,3-di-yl-bis-(azanediyl))-bis-(carbonothioyl))-dibenzamide (4) as a result of alkaline hydrolyses. We produced corresponding di-Thiocarbamide (5) at outcome percentage accordingly. The structure of the product we got resolved by electronic spectrum.
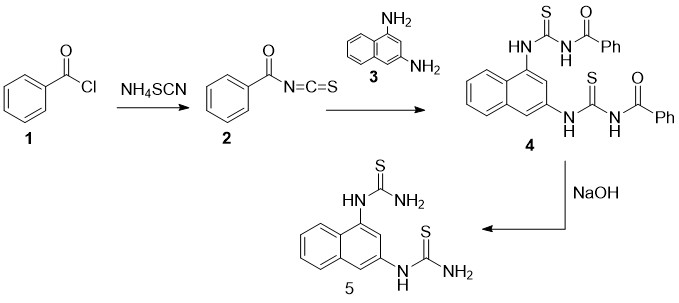
Scheme 1. Synthesis of Dithiocarbamide 5.
On the following stage we proceeded with cyclization (Scheme2) of Naphthalene di-Thiocarbamide (5). Ethyl bromoacetate has been used as cyclizating agent. Reaction was conducted at temperature of 80 0C in Ethanol environment Oxo-substances (6) are obtained in 55% (Scheme 2).
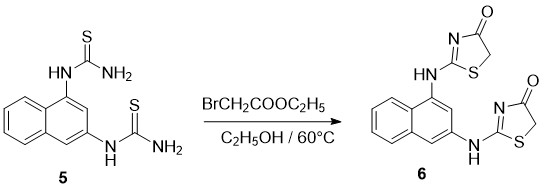
Scheme 2. Preparation of 2,2'-(naphthalene-1,3-diylbis(azanediyl))bis(thiazol-4(5H)-one)
On the third stage of work we carried out desoxychlorination. There are many descriptions of such cases in publications. As most of them are characterized with low outcomes, our aim was to find out optimal conditions of desoxychlorinationreactions in order to increase the productivity.
We carried out desoxychlorination experiment changing concentration of interactive substances, temperature and the time of the reaction. The outcome of targeted product was still low in all cases. By heating 6 substance and POCl3 mixture at 60 0C with correlation of Pyridine 1:1:1 we obtained 23% outcome of the final product (7) (Scheme 3).
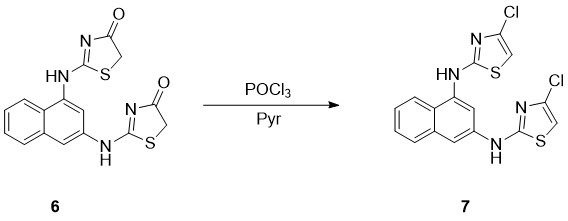
Scheme 3. Preparation of1N,3N-bis-(4-chloro-4,5-dihydrothiazol-2-yl)naphthalene-1,3-diamine
We were unsuccessful in increasing the productivity even when we replaced Pyridine with N, N-dimethylaniline.
It is known from publications that reactions of desoxychlorination are conducted in Phosphorus Oxychloride without base. In our case even in likewise conditions we got resinous mass and final product was formed only as a trace. From our point of view above mentioned results can be explained by fact that in case of exceeding amount of Phosphorus Oxychloride we may get an intermediary products O-Phosphates as well as Dimer (0,0)-Phosphates (scheme 4) [10].
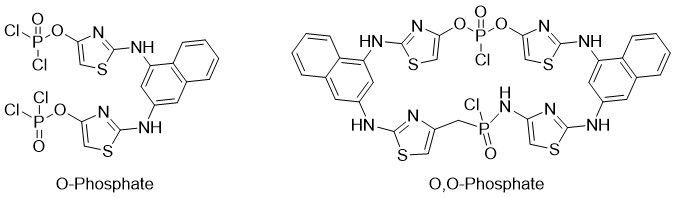
Scheme 4. Structure of intermediary products O-Phosphates and (0,0)-Phosphates
On the last stage of synthesis product 8 were obtained by nucleophilic aromatic substitution in DMF solution, using thebenzimidazole in excess as reagents and bases (Scheme 5).
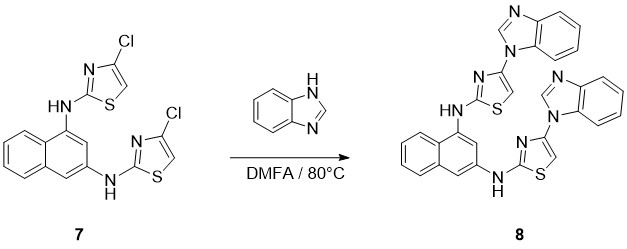
Scheme 5. Preparation of 1N,3N-bis(4-(1H-benzo[d]imidazol-1-yl)thiazol-2-yl)naphthalene-1,3- diamine
The reaction was carried out at the temperature 80 0C for as long as 24 hours at constant mixing and resulted in reaching 35 % of productivity of targeted product (8).
Was studied of a preparative method for the production of 1,3-Naphthyl di-Thiourea is approved. The optimum conditions for the reaction are established;
Was carried out Condensation of Naphthalene di-Thiocarbamide with ethyl bromoacetate corresponding to 2,2'-(naphthalene-1,3-diyl-(azanediyl))-bis-(thiazole-4(5H)-one). The optimal conditions for the cyclization reaction.Have been establishedthe oxychlorination reaction was carried out. In order to increase the yield, the optimal reaction conditions were selected: the best yield is achieved when the initial oxo-compounds, phosphorus oxychloride and pyridine are taken in equal proportions.
Condensation of 1N,3N-bis-(4-chlortiazol-2-yl)naphthalene-1,3-diamine with benzimidazole was performed. Optimal reaction conditions are established.
The date of the IR and NMR spectras are given in the experimental section.
Experimental Part
Chemical Procedures. General. NMR spectra were recorded with a BrukerAvance 400 MHz spectrometer at 300 K, using TMS as an internal standard. IR spectra (KBr) were measured with a Bruker Tensor 27 spectrometer.
2,2'-(naphthalene-1,3-diylbis(azanediyl))bis(thiazol-4(5H)-one) (6). The Naphthylthioureas (5) (27 mmol) were dissolved in 90 mL of ethanol pa at 45 °C. To this solution ethyl bromoacetate (70 mmol) was added, and the mixture was stirred at this temperature for 2 h. After cooling to room temperature, the mixture was neutralized with aqueous ammonia. Drop wise addition of water when stirring led to precipitation of the product, which was removed by filtration and dried. Yield: 55%; white crystals. Mp: 255-2570C;1H NMR (DMSO-d6): δ (ppm) )11.97 (s, 1H), 10.35 (s, 1H); 7.91(trip, 2H, J= 7.8; J= 8.2), 7.70 (d, 1H, J=8.2 ), 7.55-7.45 (m, 2H), 7.05 (d, 1H, J=7.16), 4.05 (s, 2H); 3.97 (s, 2H). 13C-NMR(400 MHz, CDCl3): 175.22; 126.44; 134.38; 128.41; 127.57; 126.88; 126.44; 126.26; 124.82; 123.45; 116.44; 34.78; IR (KBr, ν, cm -1): 3383, 1299 (N-H), 3091 (C-H), 1689 (C=O), 1562, 1470 (C=N, arom. C=C).
1N,3N-bis(4-chloro-4,5-dihydrothiazol-2-yl)naphthalene-1,3-diamine (7). A mixture of the appropriate compound 6 (4.8 mmol), 3 mL of POCl3, and 0.4 mL of pyridine was refluxed for 3 h. After the mixture was cooled to room temperature, the solvent was removed under reduced pressure. The residue was dissolved in ether, and the solution was washed twice with 5% aqueous NaOH, brine, and water. The solvent was dried (Na2SO4) and removed under reduced pressure. The crude product was purified by column chromatography (Hexane:Diethyl ether, 5:1). Yield: 23%, Mp: 1850 C, Rf= 0.35(Hexane:Diethyl ether 3:1), 1H NMR (DMSO-d6): δ (ppm) )10.33 (s, 1H), 10.28 (s, 1H), 8.20-8.18 (m, H) 8.00(d, H, J= 7.28), 7.96-7.94 (m, H), 7.72 (d, H J=8.2), 7.57-7.50 (m, 2H), 6.81 (s, H); 6.34 (s, H). 13C-NMR(400 MHz, CDCl3): 174.63; 143.91; 133.84; 127.87; 127.03; 126.35; 125.74; 124.33; 122.90; 115.97; 34.75.IR (KBr, ν, cm -1): 341, 3326, 3030, 2968, 1599, 1508, 1353, 1299, 768, 716
Synthesis of Target Derivates 8. The appropriate compound 7 (3.6 mmol) and the benzimidazole (36 mmol) were stirred in 7.5 mL of DMF at 80 °C for 2-8 days (TLC control). The mixture was cooled to room temperature and poured into 300 mL of 5% aqueous Na2CO3. The crude product was filtered off, dried, and purified by column chromatography (SiO2, ethyl acetate). Yield: 35%; Mp: 2720 C, Rf= 0.40(Hexane:Diethyl ether 3:1),
1H NMR (DMSO-d6): δ (ppm) ) 10.51 (s, 1H), 10.28 (s, 1H), 8.55 (dd, 2H), 8.08 (d, 2H); 7.99-8.00 (m, 4H); 7.21-7.31 (m, 7H); 6.79 (s, 1H); 6.50 (s, 1H); 5.78 (s, 1H);13C-NMR(400 MHz, CDCl3): 174.63; 143.91; 134.67; 133.84; 130.50; 128.15; 127.87; 127.03; 126.35; 126.26; 125.90; 125.74; 124.33; 122.90; 120.47; 119.67; 115.97; 34.75.IR (KBr, ν, cm -1): 1585, 1630, 1700, 3225.
References
- SiavoshMahboobi, Andreas Sellmer, HeymoHo¨cher, EmerichEichhorn, Thomas Bar, Mathias Schmidt, Thomas Maier, Josef F. Stadlwieser, and Thomas L. Beckers.4-(Imidazol-1-yl)thiazol-2-yl]phenylamines. A Novel Class of Highly Potent Colchicine SiteBinding Tubulin Inhibitors: Synthesis and Cytotoxic Activity on Selected Human Cancer Cell Lines Med. Chem. Vol. 49, pp. 5769-5776, 2006.
- Beckers, T.; Mahboobi, S. Natural, semisynthetic and syntheticmicrotuble inhibitors for cancer therapy. Drugs Future, Vol. 28, 767-785, 2003.
- Byth, K. F.; Cooper, N.; Culshaw, J. D.; Heaton, D. W.; Oakes, S.E.; Minshull, C. A.; Norman, R. A.; Pauptit, R. A.; Tucker, J. A.;Breed, J.; Pannifer, A.; Rowsell, S.; Stanway, J.J.; Valentine, A. L.;Thomas, A. P. Imidazo[1,2-b]pyridazines: a potent and selektiveclassofcyclin-dependent kinase inhibitors. Med. Chem. Lett., Vol. 14, pp.2249-2252, 2004.
- Sabrin R. M. IbrahimGamal A. Mohamed. Naturally occurring naphthalenes: chemistry, biosynthesis, structural elucidation, and biological activities. Phytochemistry Reviews. Vol. 15, 279-295, 2016.
- Scott, David A.; Aquila, Brian M.; Bebernitz, Geraldine A.; Cook, Donald J.; Dakin, Les A.; Deegan, Tracy L.; Hattersley, Maureen M.; Ioannidis, Stephanos; Lyne, Paul D.Pyridyl and thiazolylbisamide CSF-1R inhibitors for the treatment of cancer. Bioorganic & Medicinal Chemistry Letters. 18 (17): pp. 4794-4797, 2008.
- Golankiewicz, P. Januszczyk, M. Gdaniec and Z. Kosturkiewicz. Reaction of acylaminocyanoesters with 2,4-bis (4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane 2,4-disulfide leading to substituted aminothiazoles.A Tetrahedron, Vol.41(24) pp.5989-5994. 1985.
- Muhammad, H.; Muhammad, A.H.N.; Maria, V.B.; Jamshed, I.; Alexander, R.;Bernhard, K.K.; Christian, G.H. 1,3,1',3'-(Dinaphthalene-1,8-diyl)bisthiourea. Molecules, Vol. 19, pp. 8080-8092.
- 8. Muthu, K.; Meenatchi, V.; Rajasekar, M.; Aditya, P.; Meena, K.; Agilandeshwari, R.;Kanagarajan, V.; Meenakshisundaram, S.P. Combined theoretical and experimental studies on the molecular structure, spectral and Hirshfeld surface studies of NLO tris(thiourea)zinc(II) sulfate crystals.J. Mol. Struct. Vol. 1091, pp. 210-221.2015.
- Sivakumar, N.; Kanagathara, N.; Varghese, B.; Bhagavannarayana, G.; Gunasekaran,S.; Anbalagan, G. Structure, crystal growth, optical and mechanical studies of poly bis (thiourea) silver (I) nitrate single crystal: A new semi organic NLO material. ActaA, Vol. 118, pp. 603-613.2014.
- Euan A. Arnott, Lai C. Chan, Brian G. Cox, Brian Meyrick, and Andy Phillips. POCl3 Chlorination of 4-Quinazolones. JOC, Vol. 76, pp. 1653-1661, 2011.
Recieved: 06-04-2021 | Web published: 30-04-2021 | Views 1893


