J. Geo. Chem. Soc., 2022, Vol. 2, Issue: 1, pp. 0 - 0
Spectrophotometric determination of promethazine hydrochloride with sodium persulphate in drug release experiment in vitro
Abstract. A rapid and simple spectrophotometric procedure for the determination of promethazine hydrochloride in buffer solution is proposed via the oxidation of the drug. The promethazine hydrochloride is oxidized with sodium persulphate in acidic medium, which leads to form a dark pink to reddish color product, that has a maximum absorption at 532 nm. Influence of different persulphates acidity of the reaction medium and concentration of sodium persulphate were studied in order to enhance the sensitivity of the proposed method. It was found that Beer’s law is obeyed within the concentration range of 5.0 – 80.0 μg/mL, with 2.0 μg/mL of detection limit. The developed method was applied for estimation of released promethazine hydrochloride from microemulsion systems.
Keywords: sodium persulphate, promethazine hydrochloride, pH of reaction medium
Introduction
Promethazine hydrochloride, l0-(2-(dimethyl-amino) propyl) phenothiazine monochloride (PMT), is a drug frequently used in pharmaceutical preparations. It is widely used in the treatment of
several mental disorders from moderate to severe illnesses such as schizophrenia. The drug is commonly known as neuroleptic tranquilizer and often used as an antihistamine, sedative, antiemetic, analgesic, anticholinergic, anti-motion sickness and anesthetic medication.
Several methods have been reported for the quantitative determination of promethazine hydrochloride in pure solution and in pharmaceutical preparations. However spectrophotometric methods because of its simplicity, high sensitivity and low cost nowadays is the preferred methodology for most routine analysis [1-9]. They mainly are devoted to the determination of PMT in both in bulk and in pharmaceutical preparations [1-3; 5-9]. Determination of PMT in phosphate buffer saline pH 7.4 is described and it can be used to estimate the amount of PMT penetrated and dissolved in the blood vessels in vitro by penetration study [4].
The present work is devoted to the development of analytical spectrophotometric method for determination of promethazine hydrochloride in buffer solution during drug release experiment in vitro. The method is depended on reaction of promethazine hydrochloride with sodium persulphate, the reaction product is dark pink to reddish color that absorbed at 532 nm.
Experimental
Chemicals and reagents
Surfactants Polyoxyethylene (20) cetyl ether (Brij-58) and Polyethylene glycol tert-octylphenyl ether (Triton X-100) and promethazine hydrochloride [10-(2-dimethylamonipropyl) phenothiazine hydrochloride (PMT) were kindly offered by Sigma-Aldrich, USA. Cellulose dialysis tubing was purchased from Aldon Corporation (USA).
Apparatus
Absorption spectra in the UV-visible region were taken on an Optizen POP UV-visible spectrophotometer using quartz cells with a path length of 1 cm. Absorption of solutes were measured on KFK-2 photoelectrocolorimeter with wavelength range 315-980 nm. Receptor buffer solution was stirred with magnetic stirrer (INTLLAB).
Drug release experiment
The micro emulsion or pure water containing the drug was placed in a dialysis tube made of cellulose (6.4 mm × 10 mm) and immersed into 50 ml of phosphate buffer with pH7.4 (0.0075 M Na2HPO4 and 0.0025 M NaH2PO4 regarding water) at a constant temperature of 30°C. The system was stirred with a magnetic stirrer. Aliquots of 1 ml were taken after 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210, 240 min. The same amount of fresh phosphate buffer was added to the receptor solution each time to maintain the dissolution volume.
Determination of promethazine hydrochloride
To each aliquot (1 mL) of promethazine hydrochloride in buffer solution 0.75mL of 1% sodium persulphate was added and then left for half an hour to stabilize the intensity of the pink to reddish color. A dark-pink to reddish color resulted which was diluted up to 10 mL with distilled water. The absorbance of this red solution was measured at 532 nm. The experiment was repeated with different concentrations of promethazine hydrochloride and a calibration curve was plotted. The color reaction obeyed Beer’s Law in the concentration range 5-80 μg/mL of promethazine hydrochloride.
Results
Influence of different persulphates
In order to create the optimal conditions necessary for the rapid and quantitative formation of a colored product in a buffer solution (pH 7.4) with maximum stability and sensitivity, various persulphates were applied for the oxidation reaction, e.g. ammonium, sodium and potassium persulphates. It has been established that the maximum color intensity is achieved by treatment with sodium persulphate, while ammonium persulphate does not give stable coloration, and potassium persulphate does not oxidize promethazine at all. To do this, we compared the pH values of solutions of ammonium, sodium, and potassium persulphates in water, in a buffer, and also in a solution of promethazine in a buffer (Figure 1). A significant effect of pH on the color intensity was revealed. The addition of persulphates to the buffer solution increases the pH value (B, Figure 1) of reaction medium compared to their values in water (A, Figure 1). The addition of promethazine (C, Figure 1) to the buffer and oxidizing solution does not change the pH of the reaction medium. As can be seen from the Figure 1, an optimal pH of 3.0 in the reaction medium for the oxidation of promethazine hydrochloride is created only in the case of adding an oxidizing solution of sodium persulphate to a phosphate buffer solution (pH 7.4).
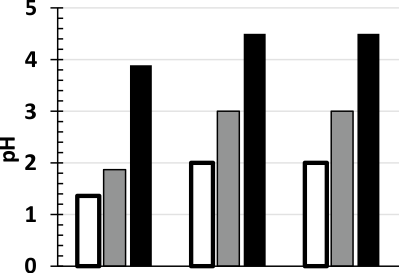
Figure 1. Diagram of pH of 1% solution of ammonium (□), sodium () and potassium (■) persulphates in water (A), buffer (B) and promethazine + buffer solution (C)
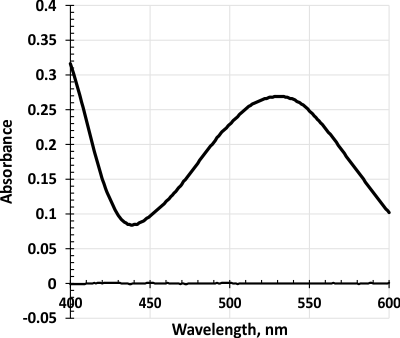
Figure 2. Absorption spectrum of the oxidized product (λmax=532 nm).
The absorption spectrum of the colored product obtained after oxidation with 1% sodium persulphate solution shows that the absorption maximum is 532 nm (Figure 2).
The study of the influence of the concentration of sodium persulphate in the oxidizing solution on the optical density showed that the coloring of promethazine hydrochloride was more intense when treated with 1% sodium persulphate solution (Figure 3). The colored product is stable or retains its color for 12 hours at room temperature. The calibration curve is linear in the range of promethazine hydrochloride concentrations within 5.0-80.0 µg/ml. Limit of detection 2.0 µg/ml.
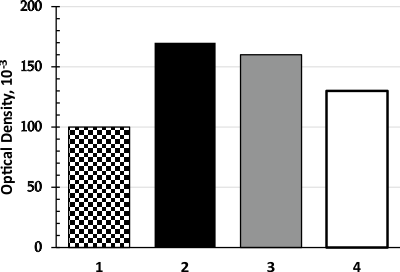
Figure 3. The diagram of the optical density of the oxidation product on the concentration of sodium persulphate (1-0.5%, 2-1%, 3-1.5% and 4-2%).
The release profile of PMT from oil-in-water microemulsions was studied. As seen from Figure, the drug release rate of PMT from microemulsions prepared on the basis of various surfactants differ from each other. Namely, concentration of released drug from Brij-58 microemulsion (curve 2, Figure 4) is higher than from Triton X-100 o/w microemulsion (curve 1, Figure 4).
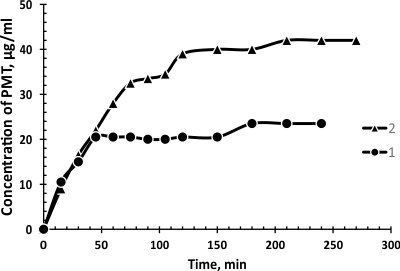
Figure 4. Concentration-time curves for promethazine hydrochloride released from 40 mM TritonX100 (curve 1) and 40 mM Brij-58 (curve 2).
The developed method for the determination of promethazine hydrochloride in a buffer solution (pH 7.4) does not require additional procedures such as heating, extraction, sample concentration. It is simple, fast, and can be successfully used in drug release experiments from complex matrixes as microemulsions or gels.
References
[1] M.M.M. Al-Rufaie, "A Sensitive Spectrophotometric Method for Trace Amounts Determination of Promethazine in Drug Formulations via Ion Pair Complex Formation," Malaysian Journal of Science, vol. 40, no. 1, pp. 80-92, 2021.
[2] M.B. Devani, B.N. Suhagia, S.A. Shah, "Specrophotometric Determination of Promethazine Hydrochloride in Burk Powder and in Its Dosage Forms," Indian J. Pharm.Sci, vol. 61, no. 2, pp. 110-112, 1999.
[3] K.H.Al-Saidi, R.A.Hammza, "Specrophotometric Determination of Promethazine Hydrochloride and Paracetamol in Pharmaceutical Tablets," Jourmal of Al-Nahrain University, vol. 17, no. 1, pp. 14-23, 2014.
[4] D. Nurahmanto, "Development and Validation of UV Spectrophotometric Method for Quantitative Estimation of Promethazine HCl in Phosphate Buffer Saline pH 7.4," Nurahmanto, Intermational Current Pharmaceutical Journal, vol. 2, no. 8, pp. 141-142, 2013.
[5] S.S. Mahmood, O.A. Hashem, M.M. Khither, "Determination of Promethazine Hydrochloride in Pharmaceutical Forms by Spectrophotomeric Method," Journal University of Kerbala, vol. 16, no. 3, pp. 28-38, 2018.
[6] H.A. Qader, N.A. Fakhre, "Spectrophotometric determination of Promethazine Hydrochloride in Pure and Pharmaceutical Dosage Forms," ZANCO Journal of Pure and Applied Sciences, vol. 29, no. 4, pp. 107-114, 2017.
[7] M.J.Saif, J.Anwar, "A New Specrophotometric Method for the Deremination of Promethazine-HCl from Pure and Pharmaceutical Preparations," Talanta, vol. 67, pp. 869-872, 2005.
[8] S.P.El-Shabouri, A.F.Youssef, F.A. Mohamed, A.I.Rageh, "Selective Specrophotometric Determination of Promethazine Pydrochloride and Thiethylperazine Maleate in Pharmaceutical Preparation," Bull. Pharm. Sci,Assiut University, vol. 8, no. 2, pp. 70-87, 1985.
[9] R.M.M. Taqi, R.J.M. Al-Timimi, M.M.Hasan, M.J.Hamzah, "Spectrophotometric Determination of Promethazine HCl in Pure and Dosage Forms," Journal of Biotechnology Research Center, vol. 13, no. 1, pp. 52-57, 2019.
Recieved: 17-01-2023 | Web published: 11-05-2022 | Views 1935


