J. Geo. Chem. Soc., 2021, Vol. 1, Issue: 1, pp. 32 - 35
Synthesis of some new azo dyes on the base of 6-aminocoumarine
Agricultural University of Georgia
Abstract. Diazotization and azo coupling reactions are versatile tools of fine organic synthesis. Azo dyes have very interesting physical, spectral and chemical properties and are widely used in the different fields of the science and technology. Moreover, some of them have very significant biological activity and are used in the healthcare or medical diagnostics. Coumarin belongs to chromene type dyes, but it may be used as an azo partner in the azo coupling reaction for the synthesis of azochromenes dyes. In the current research we have obtained four new dyes bearing two p-conjugated azo and chromene chromophores. The target compounds were synthesized by diazotizaton of 6-aminocarine (1) and with following azo coupling to 2-hydroxybenzoic acid (3a), naphthalen-2-ol (3b), 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid (3c) and (E)-3-(4-hydroxyphenyl)acrylic acid (3d). Obtained azo dyes have been used for dyeing wool fiber and variuos technical and spectral properties have been sdudied.
Keywords: azo dyes, diazotization, azo coupling, dyeing, wool
Introduction
Diazotization and azo coupling reactions are versatile tools of fine organic synthesis. These consecutive reactions commonly are used for the generation of azo chromophore and colored materials, having ability to color other substances. Azo dyes have very interesting physical, spectral and chemical properties and are widely used in the different fields of the science and technology. Moreover, some of them have very significant biological activity and are used in the healthcare or medical diagnostics. Azo dyes usually are in close contact to human body and potentially may penetrate into organism in some quantity and undergo metabolism process forming mutagenic and toxic primary aromatic amines. Therefore, it is very important to choose the non-toxic, eco- or biofriendly diazo and azo partners during azo compound construction. On the other hand, the conjunction strategy of different chromophores into one molecule structure for the aim of physical, chemical and biological properties synergizes, is another modern technique in the design of the dyes.
Coumarin belongs to chromene type dyes, but it may be used as an azo partner in the azo coupling reaction for the synthesis of azochromenes dyes [1, 2]. While 6-aminocoumarin easily reacts to sodium nitrite in the presence of hydrochloric acid and gives corresponding diazonium salt, able to couple aromatic substances and form azo compounds. A series of sulfocoumarin-, coumarin-, and 4-sulfamoylphenyl-bearing indazole-3-carboxamide hybrids with the selective Inhibition properties of tumor-associated carbonic anhydrase isozymes IX and XII have been synthesized by S. Angapelly and co-workers [3]. Moreover, coumarin azo derivatives which may be used as a dyes [4], fluorescent probes [5], antimicrobial [6]- - [7] [8] [9], gelling [10], antithrombotic [11] agents, Xa inhibitors [12], etc [13].
Results and Discussion
In the current research we have obtained four new dyes bearing two p-conjugated azo and chromene chromophores. The target compounds were synthesized in accordance with two sequential stages of diazotization-azo coupling, as shown on scheme 1. 6-aminocoumarin (1) was chosen as the diazo partner, and 2-hydroxybenzoic acid (3a), naphthalen-2-ol (3b), 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid (3c) and (E)-3-(4-hydroxyphenyl)acrylic acid (3d) for azo partners.
Diazotization of 1 has been carried out in the diluted hydrochloric acid media by the action of sodium nitrite at 0-5 °C under stirring for a period of 60 min. The finishing of the process was checked by the positive test on starch-iodine paper (generation of blue color indicates the excess of nitrous acid). The excess of nitrous acid has been removed by the addition of solid urea until terminating gas evaluation from the reaction mass. Finally, diazonium salt in the form of water solution was filtered off quickly on the filter paper in the ice bath for avoiding decomposing of 2. Purified solution of 2 has been used immediately in the azo coupling reaction with preliminarily prepared and cooled to 0°C alkali solutions of azo partners 3a-d.
The azo coupling reactions were carried out by adding diazo partner solution to the azo partner solution under vigorous stirring and careful monitoring of pH value. To convert phenolic and naphthol compounds into more reactive forms of phenolates and napholates, it is imperative to maintain a slightly alkaline pH value of about 9-10. Thus, a constant pH was adjusted from time to time by adding a 10% NaOH solution throughout the azo coupling process. The final compounds have been precipitated by adding 10% hydrochloric acid solution to pH value of 7 and isolated by filtration. The solid remains have been washed out by cold water on the filter paper, transferred to Petri dish and dried at ambient temperature in the vacuum.
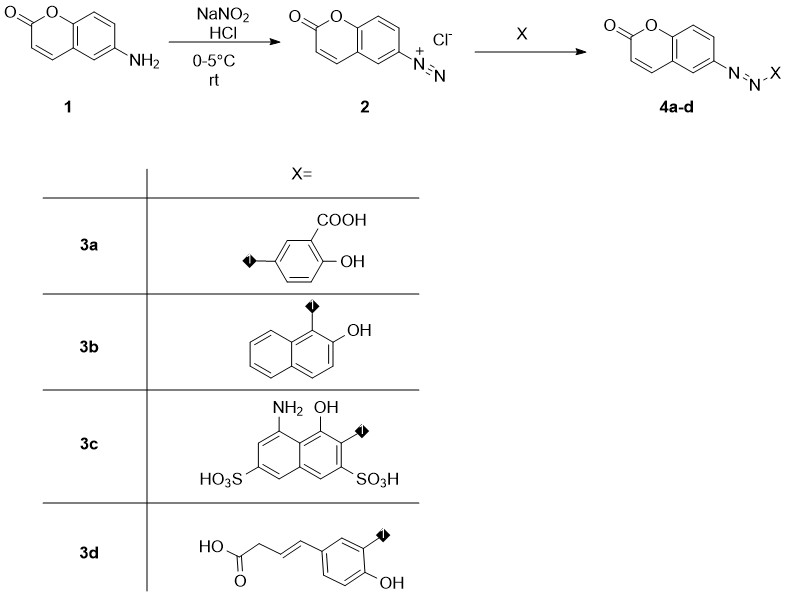
scheme 1. Synthesis of azo-coumarin dyes by diazotization-azo coupling reactions
The isolated azo dyes have been used for dyeing without additional purifications except of 4d, which was recrystallized in 1% HCl solution. The yields of final products were 40-55% (table 1, fig. 1.).
Analysis of the UV-vis spectra shows, that all obtained dyes have absorption in the visible range. 4c absorbs on the 520 nm wavelength because of longest p-conjugated system and bearing two strength electron donating groups (OH and NH2). The absorption of 4d is bathochomically shifted in comparison of 4a absorption value which caused by participation of exocyclic double bond in the p-conjugated system.
Table 1. The yields and properties of 4a-d
| N | Dye | Yield, % | lmax(e), nm, solvent | Dye uptake, % | Lab-coordinates |
| 1 | 4a | 55 | 440 (1.50´105), water | 80 | L=78, b=1, b=62 |
| 2 | 4b | 53 | 480 (1.486´105), ethanol | 60 | L=50, b=31, b=56 |
| 3 | 4c | 54 | 520 (1.9412´105), water | 75 | L=11, b=27, b=2 |
| 4 | 4d | 40 | 460 (1.7647´105) water | 74 | L=78, b=1, b=70 |
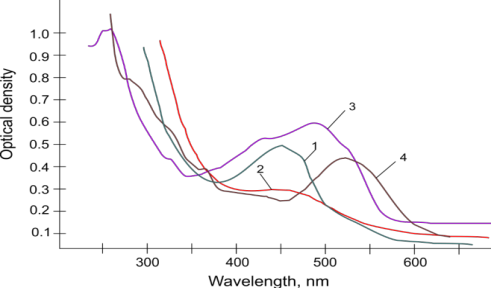
Fig. 1. UV-vis spectra: 1 – 4a, 2- 4b, 3-4c, 4- 4d
Coumarin-Azo dyes have been used for dying of wool fiber in accordance with the method described by M. Toussirot et al [14]. The preliminarily washed up, dyed and weight up fiber was put and soaked in pre-dyeing bath, containing potassium aluminium sulfate (0.3% weight of fiber). The temperature of the bath was increased up to 60°C and kept for 45 min. Then the dying bath was cooled to room temperature. Mordanted fiber was washed out with tap water to remove the excess of potassium aluminium sulfate.
For the dyeing bath, a M:L (material to liquor ratio) at 1:40 was used. The mordanted wrung out fiber was put in the dyeing bath and heated again up to 60 °C for a period of 45 min with manual periodical stirring. The fiber in bath were allowed to cool down, and then rinsed with tap water and left for drying at room temperature.
Dye uptake properties of all dyes 4a-d have been determined. For this aim, the absorbance of the dyeing bath have been measured before and after dyeing. The dyebath was cooled to room temperature prior to measures. The percentage of dye exhaustion (DE) was calculated according to the given formula:
DE = [(A0 - A1)/A0] ´ 100
where A0 and A1 are absorbances of the dye bath, before and after dyeing respectively.
The color strength and color depth of the dyed samples were determined in CIELab coordinates. L* corresponds to the brightness (100 % white, 0 – black, a* is red-green balance (+a* = red, -a* = green) and b* is the yellow-blue balance (+b* = yellow, -b* = blue). For the aim of color coordinate measurement, the high-resolution standardized screen has been used with graphical software. The dyed samples have been positioned on graphical square of the horizontally located screen surface. The color of the graphical square was changing programmatically until achieving corresponding color and the Lab-coordinate values have been recorded (see table 1).
The dyed samples have been studied light fastness and stability against wet treatment. For the light-fastness test 4 cm2 dyed fiber sample was places of the white surface, irradiated by UV light for 2 hours and re-measured the color according to above described method. Finally, the fastness has been calculated using the following relationship:
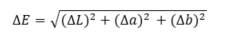
where DL =L*after - L*before; Da = a*after - a*before; Db = b*after - b*before
The fastness against wet treatment has been performed and resistance against extraction by Soap, HCl, NaOH and ethanol have been carried out. The color changes have been calculated as described above and the results are listed in the table 2.
Table 2. Light fastness and stability against wet treatment of 4a-d
| N | Dye | Light fastness % | Soap, 5% | HCl, 5% | NaOH, 5% | Organic Solvents (EtOH) |
| 1 | 4a | 90 | 94 | 92 | 86 | 94 |
| 2 | 4b | 85 | 93 | 92 | 88 | 94 |
| 3 | 4c | 80 | 91 | 90 | 81 | 96 |
| 4 | 4d | 93 | 95 | 93 | 87 | 94 |
Experimental Section
All of the chemicals used were of commercial grade and were further purified be recrystallization and redistilled before use. The solvents were spectroscopic grade. UV-Vis absorption spectra were measured on spectrophotometer Shimadzu UV-1900 (Japan). Fastness to light, sublimation, respiration and wash fastness were assessed in accordance with valid state standards [15, 16]. The dyeing of wool fiber was carried out and exhaustion of the dyed fibers was determined according to the literature [17].
2-oxo-2H-chromene-6-diazonium chloride (2). 1.24 mmol (0.2 g) 6-aminocoumarin (1) and 1.46 ml 10% hydrochloric acid was placed in the chemical beaker, equipped with magnetic stirrer and thermometer, and dissolved. After complete dissolving of amine 1, the reaction mass was cooled up to 0°C by using ice-water bath. 1.3 mmol (0.09 g) NaNO2 in 2 ml water was added dropwise for a period of 30 min under vigorous stirring and keeping temperature below 5°C. The excess of nitrous acid was checked after 30 min of complete addition of NaNO2 solution and was removed by addition of solid urea in the case of necessarily. Finally, the solution of 2 was filtered quickly and used immediately in the azo coupling reaction.
Azo coupling reaction (General method). The solution of 2 was added dropwise to a solution of 3a-d (1.20 mmol) in minimal amount of sodium hydroxide (10%, 25 mL) over a period of 15 min with constant vigorous stirring. The reaction mixture was further stirred for a period of 1 hour and neutralized with hydrochloric acid (10%). The solids, formed after neutralization, was filtered, washed with water, dried and crystallized from ethanol.
4a: Yield 0.66 mmol (0.204 g, 55%). lmax(e), nm, solvent: 440 (1.50´105), water. Yellow crystals (L=78, b=1, b=62).
4b: Yield 0.64 mmol (0.201 g, 53%). lmax(e), nm, solvent: 480 (1.486´105), ethanol. Dark reddish-brown crystals (L=50, b=31, b=56).
4c: Yield 0.65 mmol (0.318 g, 54%). lmax(e), nm, solvent: 520 (1.9412´105), water. Dark brown crystals (L=11, b=27, b=2).
4d: Yield 0.48 mmol (0.168 g, 40%). lmax(e), nm, solvent: 460 (1.7647´105) water. Dark yellow crystals (L=78, b=1, b=70).
Conclusion.
We are able to suggest that 6 -aminocoumarin may be used as a diazo partner in the azo coupling reaction for obtaining dyes, bearing both coumarin and azo chromophores and characterized good spectral and technical properties.
References:
[1] M. Nikpassand, L. Z. Fekri, N. Changiz and F. Imani, "Synthesis of new 3-cyanocoumarins with C-6 azo function using ultrasound and grinding techniques in the presence of nano Fe3O4," Letters in Organic Chemistry, vol. 11, no. 1, pp. 29-34, 2014.
[2] O. K. Rasheed and P. Quayle, "Azo Dyes: New Palladium- and Copper-Catalyzed Coupling Reactions on an Old Template," Synthesis, vol. 50, no. 13, pp. 2608-2616, 2018.
[3] S. Angapelly, P. V. Sri Ramya, A. Angeli, C. T. Supuran and M. Arifuddin, "Sulfocoumarin-, Coumarin-, 4-Sulfamoylphenyl-Bearing Indazole-3-carboxamide Hybrids: Synthesis and Selective Inhibition of Tumor-Associated Carbonic Anhydrase Isozymes IX and XII," ChemMedChem, vol. 12, no. 19, pp. 1578-1584, 2017.
[4] R. Amjad, M. A. Munawar, S. R. Khan and M. Naeem, "Synthesis and spectral studies of some novel Coumarin based disperse azo dyes," Pakistan Journal of Scientific and Industrial Research, vol. 52, no. 3, pp. 117-121, 2009.
[5] S. Guha, S. Lohar, I. Hauli, S. K. Mukhopadhyay and D. Das, "Vanillin-coumarin hybrid molecule as an efficient fluorescent probe for trace level determination of Hg(II) and its application in cell imaging," Talanta, vol. 85, no. 3, pp. 1658-1664, 2011.
[6] B. P. Choudhari and V. V. Mulwad, "Synthesis and antimicrobial screening of N-[coumarin-6-ylamino]thiazolidinone and spiro indolo-thiazolidinone derivatives," Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, vol. 44B, no. 5, pp. 1074-1078, 2005.
[7] V. V. Mulwad and S. A. Mayekar, "Synthesis and antimicrobial screening of 5-(4,7-dimethyl-2-oxo-2H-benzopyran-6-ylazo)-2-methyl-6-morpholin-4-yl-2,3-dihydro-3H-pyrimidin-4-one and 5-(4,7-dimethyl-2-oxo-2H-benzopyran-6-ylazo)-2-methyl-6-piperidin-1-yl-2,3-dihydro-3H-pyrimidin-4-one," Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, vol. 46B, no. 11, pp. 1873-1878, 2007.
[8] B. P. Choudhari and V. V. Mulwad, "Synthesis and antimicrobial screening of 3H,11H-9-methyl-3-oxopyrano[2,3-f]cinnolino[3,4-c]pyrazole and its derivatives," Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, vol. 45B, no. 1, pp. 309-313, 2006.
[9] V. V. Mulwad, Y. Mhamunkar and B. Langi, "Synthesis and biological activity of heterocycles synthesized from diazo compounds," Indian Journal of Heterocyclic Chemistry, vol. 19, no. 4, pp. 349-352, 2010.
[10] H. Okamoto and Y. Morita, "Alkoxyphenylazocoumarin-type gelling agents, organic substance gels formed therewith, and their preparation". Japan Patent JP2012017384, 26 01 2012.
[11] K. M. Amin, N. M. Abdel Gawad, D. E. Abdel Rahman and M. K. M. El Ashry, "New series of 6-substituted coumarin derivatives as effective factor Xa inhibitors: Synthesis, in vivo antithrombotic evaluation and molecular docking," Bioorganic Chemistry, vol. 52, pp. 31-43, 2014.
[12] K. M. Amin, N. M. Abdel Gawad, D. E. Abdel Rahman and M. K. M. El Ashry, "New series of 6-substituted coumarin derivatives as effective factor Xa inhibitors: Synthesis, in vivo antithrombotic evaluation and molecular docking," Bioorganic Chemistry, vol. 52, pp. 31-43, 2014.
[13] P. Datta, D. Sardar, R. Saha, T. K. Mondal and C. Sinha, "Structure, photophysics, electrochemistry and DFT calculations of [RuH(CO)(PPh3)2(coumarinyl-azo-imidazole)]," Polyhedron, vol. 53, pp. 193-201, 2013.
[14] M. Toussirot, W. Nowik, E. Hnawia, N. Lebouvier, A.-E. Hay, A. de la Sayette, M.-G. Dijoux-Franca, D. Cardon and N. M., "Dyeing properties, coloring compounds and antioxidant activity," Dyes and Pigments, vol. 102, pp. 278-284, 2014.
[15] GOST 9733.3-083., Testing methods of dye fastness against light under artificial exposure (in Russian).
[16] GOST 9733.3-083. Testing methods of dye fastness against physical-chemical treatment (in Russian).
Recieved: 30-11--0001 | Web published: 17-06-2021 | Views 1923


