J. Geo. Chem. Soc., 2021, Vol. 1, Issue: 1, pp. 42 - 45
Study of influence of oil phase and co-surfactant on the microenvironment of polyoxyethylene (4) lauryl ether reverse micelles via methyl orange as optical probe
Ivane Javakhishvili Tbilisi State University
Abstract. The microenvironment of reverse micelles of polyoxyethylene (4) lauryl ether (Brij 30) was studied by UV-visible spectroscopy on the basis of methyl orange as molecular probe. The influence of different factors, viz. concentration of surfactant Brij-30, nature of oil phase (hexane and decane) and co-surfactant (n-butanol and n-heptanol) on the changes in electronic spectrum of methyl orange was investigated. An influence of the mentioned factors on the value of association degree of optical probe with reverse micelles were studied. Profile of dependence of methyl orange absorption maxima versus water content indicates the existence of three types of water (primary bound water, secondary bound water and free water) in the water pockets of reverse micelles.
Keywords: Brij-30 reverse micelles, Methyl orange, association degree, water pockets, co-surfactant
Introduction
A microemulsion consists of at least three components: an oil phase, an aqueous phase and a surfactant. In some cases, it also contains a fourth component, co-surfactant. Depending on the ratio of the components, various limiting types of microemulsions are selected in accordance with the microstructures, which vary from very small water droplets in the oil phase (w/o microemulsion) to small oil droplets in the aqueous phase (o/w microemulsion). Microemulsions play an important role in a person's daily life. Colloidal domains with their self-aggregating systems have long been considered the most attractive and important area of research [1-3].
Among amphiphilic molecules, nonionic surfactants are of particular industrial, biological, and physicochemical significance. Amphiphile of polyoxyethylene non-ionic surfactant is the subject of numerous studies. Special attention is paid to the non-ionic structures of micelles and their dynamic properties over the past two decades [4]. Non-ionic micelles exhibit a variety of aggregation, and their behavior differs in two-phase and three-phase systems, depending on concentration and temperature.
Once confined in the nanoscale pockets of reversed micelles, the already complex structure of water becomes more complex; therefore, the study of the properties of water droplets of reversed micelles is one of the most urgent problems today [5]. Non-ionic reversed micelles are studied by molecular methods. The microenvironment of reversed micelles are characterized using special spectral parameters, namely spin, luminescent and UV-visible probes. The interaction of optical probes with surfactants includes hydrophobic and electrostatic interactions, hydrogen bond interactions and interactions induced by Van der Waals forces [6-9]. However, the interaction of the molecular probe with the micelle surface causes changes in the electronic spectrum of the probe [7-8]. The interaction of molecules of an optical probe with reversed micelles has been studied in detail [10]. However, the mechanisms underlying the interaction of molecular probe-surfactant in reversed micelles have not been fully established [7, 11]. The study of the microenvironment of reversed micelles by ultraviolet-visible spectrometry using optical probes provides a unique opportunity to study the properties of water aggregates near the polar ends of a surfactant. This means that additional information can be obtained on the nature of the polar core of reversed micelles [7,9,11].
The aims of the presented work were: a) to study the microenvironment of reverse micelles prepared on the basis of polyoxyethylene (4) lauryl ether (Brij-30) using UV-visible spectroscopy via molecular probe methyl orange (MO); (b) evaluation the effect of both the oil phase and co-surfactant, also surfactant concentration on the degree of association of the optical probe to polyoxyethylene polar groups of Brij-30.
Experimental
Reverse microemulsions were prepared on the basis polyoxyethylene (4) lauryl ether (Brij30, Fluka, BioChemika, Switzerland), hexane, decane, n-butanol, n-heptanol and water. The Brij-30 (C12E4) has a molecular formula of C12H25(OCH2CH2)4OH (Figure 1, a). The hydrophilic-lipophilic balance (HLB) of Brij-30 is equal to 9.7 and the critical micelle concentration (CMC) is in the range of 7 to 14.52 mgL-1.
Transparent one-phase solutions were achieved through gentle shaking. For systems of low water content, the solutions became one phase quickly. All spectroscopy experiments were performed on stable, one-phase systems at room temperature.
UV-vis absorption spectra were recorded in a UV-visible spectrophotometer Optizen POP using cells with 1 cm path length. Methyl orange (MO) was used as optical probe (Figure 1, b).
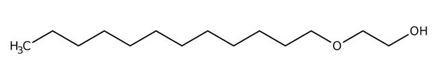
(a)
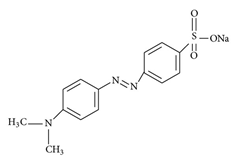
(b)
Figure 1. (a) The structure of polyoxyethylene (4) lauryl ether (Brij-30); (b) the structure of Methyl orange.
The influence of water on the association degree of methyl orange with reverse micelles was studied [9]. Association degree of MO with Brij-30 reverse micelles was calculated by absorption data of MO at wavelengths of 408 and 416 nm in 0.125 M Brij30 solution in hexane at different water/surfactant ratio (W) by equation:
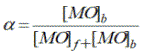 (3)
(3)
Concentrations of free and bound MO were determined by solution of equation systems at intermediate concentrations of Brij-30:
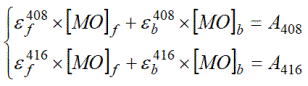 (4)
(4)
Results and Discussion
The absorption spectra of methyl orange (MO) in Brij-30 reversed micelles at different water/ surfactant ratios (W) are shown in Figure 2. Possible states of water in the polar core of a nonionic reversed micelle (water pocket or water nano pool) when water is added to the Brij-30 solution in oil include: a) water that directly interacts with the oxyethylene groups of the surfactant (primary bound water), b) bound water located next to hydrated oxyethylene groups (secondary bound water), and c) bulk water, i.e. free water.
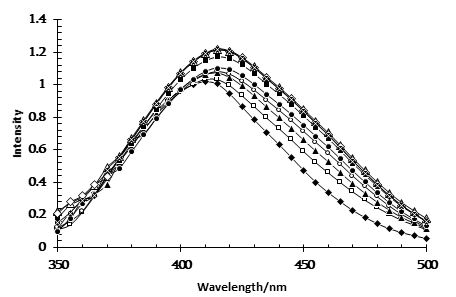
Figure 2. Absorption spectra of MO in Brij-30/heptanol/decane microemulsions at different values of W. (♦)-W=0 ; (□)- W=0.22; (▲)-W=0.44; (O) – W=0.66; (●)- W=0.88; (■)-W=1.11; (Δ) – W=1.33; (◊) - W=1.77; (x) - W=2.22. [MO]= 2.5×10-3M
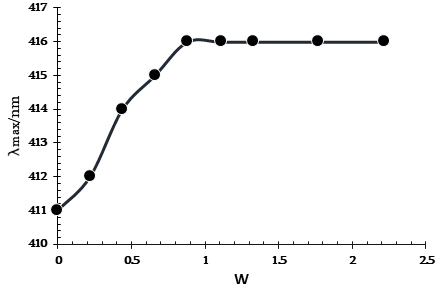
Figure 3. Variation of λmax of MO in reverse micelles Brij/heptanol/decane as a function of W.
As shown in Figure 3, the MO absorption maximum (which is a measure of micropolarity) increases from 411 nm to 414 nm (primary bound water) within 0-0.5 W. The growth of the MO absorption maximum (secondary bound water) slows down with a further increase in W until its value reaches 416 nm at W = 0.9 (water nanodroplets are formed).
The study of the effect of the nature of the oil phase on association degree of MO with polyoxyethylene groups of Brij-30 showed that in the case of n-decane, the complete release of MO occurs at a value of W = 2.2 i.e. water nanodroplets are formed at lower value of W than in the case of hexane (W = 3.5) (Figure 4, curves 3 and 4). This refers to the use of n-butanol as a co-surfactant.
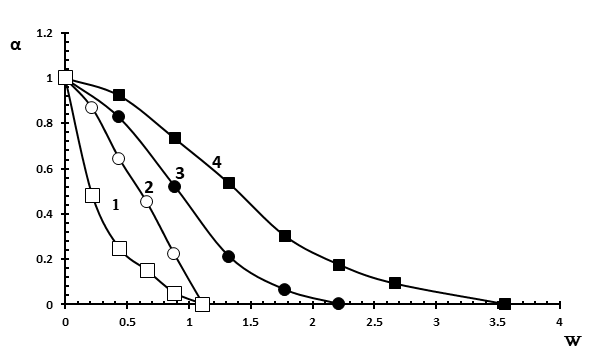
Figure 4. Dependence of the association degree of MO to Brij-30 reverse micelles on the W value.
[Brij-30]=0.125 M in the presence of hexane and butanol (■), decane and butanol (●);
[Brij-30]=0.250 M in the presence of hexane and butanol (□), decane and butanol (○).
When n-heptanol is used in combination with this system instead of n-butanol, the complete release of MO (formation of water nanoparticles) occurs in both cases at W = 0.9 (Figure 5, curves 2 and 4), i.e., the difference between W corresponding to the values of the complete release of MO is no longer observed for reversed micelles prepared on the basis of hexane and decane, which was found in the case of butanol (Figure 4, curves 3 and 4).
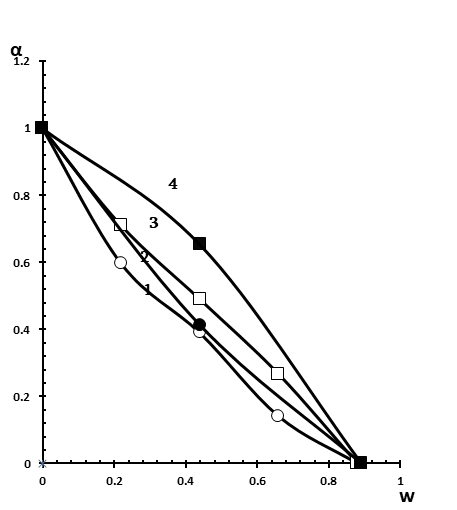
Figure 5. Dependence of the association degree of MO to Brij-30 reverse micelles on the W value. [Brij-30]=0.125 M in the presence of hexane and n-heptanol (■), decane and n-heptanol (●); [Brij-30]=0.250 M in the presence of hexane and n-heptanol (□), decane and n-heptanol (○).
As to the effect of surfactant concentration on association degree of MO with polyoxyethylene groups of Brij-30, it was found that an increase in surfactant concentration promotes the formation of aqueous nano-pockets at a lower value of W, with an oil phase in the form of hexane (Figure 4, curves 1 and 4), as well as in the case of decane (Figure 4, curves 2 and 3). This concerns to the use of n-butanol as a co-surfactant. In the case of n-heptanol as a co-surfactant, the formation of water nanodroplets occurs at the same value of W (W = 0.9) at both low (0.125 M Brij-30) and high (0.250 M Brij-30 ) surfactant concentrations (Figure 5). However, the curves of association degree of MO versus W in the case of hexane (Figure 5, curves 3 and 4) at both concentrations of Brij-30 are arranged under the curves obtained in the case of n-decane (Figure 5, curves 1 and 2).
Conclusions
- The structure of reversed micelles of polyoxyethylene (4) lauryl ether was studied by UV-vis spectroscopy using n-hexane and n-decane as oil phases, n-butanol and n-heptanol as a co-surfactant, and methyl orange as a molecular samples.
- The absorption maximum of an optical probe of methyl orange in reversed micelles is shifted bathochromically within 407-416 nm with an increase in the water / surfactant molar ratio.
- It was found that the formation of water nano pockets in reverse microemulsions in n-decane medium begins at a lower water / surfactant molar ratio (W = 2.2) compared to hexane (W = 3.5), at a fixed surfactant concentration of 0.125 M Brij-30.
- It was found that the association degree of methyl orange with reversed micelles of Brij-30 decreases less with an increase in the water / surfactant molar ratio in the presence of butanol as a co-surfactant than with heptanol.
References
[1] S.P. Moulik, A.K. Rakshit, „Physicochemistry and Applications of Microemulsions“ Journal of Surface Science Technology. Vol. 22, No.3-4, pp. 159-186, 2006.
[2] B.K. Paul, S.P. Moulik, „Uses and applications of microemulsions“, Current Science. Vol.80, No. 80, pp. 990-1001, 2001.
[3] R. Najjar, „Microemulsions - A Brief Introduction. In: Microemulsions – An Introduction to Properties and Applications“. InTech. pp. 3-30, 2012.
[4] B. Kronberg, K. Holmberg, B. Lindman. „Surfactants and Polymers Containing Oxyethylene Groups Show a Complex Behavior“, Surface Chemistry of Surfactants and Polymers, First Edition, John Wiley & Sons, Chapter 7, pp. 345-387, 2014.
[5] M.L. Arsene, I. Raut, M. Calin, M.L. Jecu, M. Doni, A.M. Gurban. „Versatility of Reverse Micelles: From Biomimetic Models to Nano (Bio)Sensor Design“. Processes, Vol. 9, pp. 345, 2021.
[6] D. Zhu, Z.A. Schelly, „Investigation of the Microenvironment in Triton X-100 Reverse Micelles in Cyclohexane,Using Methyl Orange as a Probe“ Langmuir, Vol. 8, pp. 48-50, 1992.
[7] A.R. Petcu, E. A. Rogozea, C. A. Lazar, N.L. Olteanu, A. Meghea, M. Mihaly, „Specific interactions within micelle microenvironment in different charged dye/surfactant systems“, Arabian Journal of Chemistry, Vol. 9, pp. 9-17, 2016.
[8] N.M. Correa, M.A. Biasutti, J.J. Silber, „Micropolarity of Reversed Micelles: Comparison between Anionic,Cationic and Nonionic Reversed Micelles“ Journal Of Colloid And Interface Science, Vol. 84, No. 0653, pp. 570-578, 1996.
[9 ] L. Qi, J. Ma, „Investigation of the Microenvironment in Nonionic Reverse Micelles Using Methyl Orange and Methylene Blue as Absorption Probes“ J Colloid Interface Sci. Vol. 197, pp. 36-42, 1998.
[10] J.J. Silber, A. Biasutti, E. Abuin, E. Lissi, „Interactions of small molecules with reverse micelles“, Advances in Colloid and Interface Science, Vol. 82, pp.189 -252, 1999.
[11] M.E. MAXIM, G. STNG, A. IOVESCU, A. BRN, C. ILIE, D.F. ANGHEL, „Monitorizing Methylene Blue Inclusion In Reverse Micellar Nanostructures“, Rev. Roum. Chim., Vol. 57 No 3, pp. 203-208, 2012.
Recieved: 10-05-2021 | Web published: 17-05-2021 | Views 1682


